Adeno-associated virus (AAV) analytics

Our group is expanding the boundaries of viral vector analytical methods to improve process characterization for gene therapies. We are developing analytical methods for product characterization using a variety of techniques such as bio-layer interferometry, peptide mapping by LC-MS, transmission electron microscopy (TEM), and quantitative and droplet digital PCR.
Monoclonal antibody (mAb) product quality analysis
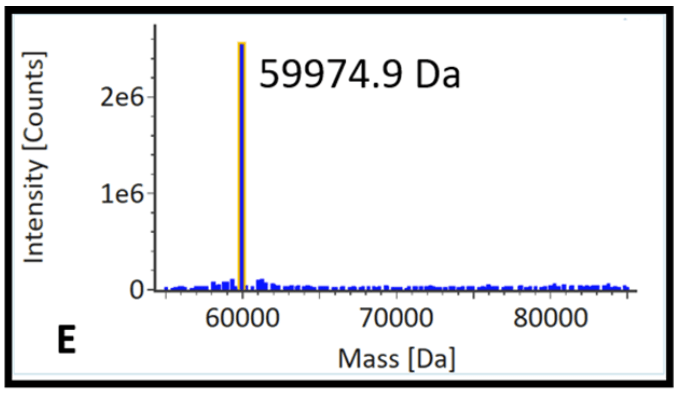
Variability in product quality attributes (PQAs) such as post-translational modifications (PTM) and residual host cell protein (HCP) profiles of a mAb product can affect safety and efficacy. Our group and many others have observed that process parameters, culture conditions, and media additives can affect these PQAs and many others. We have recently been interested in using LC-MS methods to study how glycan profiles of mAbs change in response to changes in process conditions and how HCP profiles change during CHO cell aging. We also discovered that lipoprotein lipase is a difficult to remove HCP and observed that its presence in drug product can lead to stability concerns in mAb products.